16 November 2023
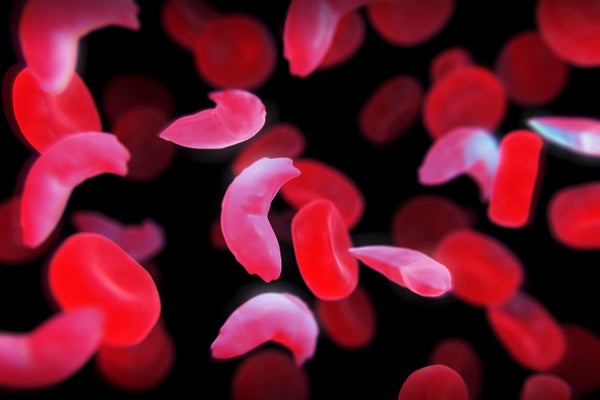
The approval by the Medicines and Healthcare products Regulatory Agency (MHRA) follows promising results from clinical trials that tested the one-time treatment, which is administered by intravenous infusion and was developed by Vertex Pharmaceuticals in Boston, Massachusetts, and CRISPR Therapeutics in Zug, Switzerland.
The trial for sickle-cell disease has followed 29 out of 45 participants long enough to draw interim results. Casgevy completely relieved 28 of those people of debilitating episodes of pain for at least one year after treatment.
Researchers also tested the treatment for a severe form of β-thalassaemia, which is conventionally treated with blood transfusions roughly once a month. In this trial, 54 participants received Casgevy and 42 patients have participated for long enough to provide interim results. For at least one year after treatment, 39 participants, or 93% of those treated, did not need a red-blood-cell transfusion. The remaining three people had their need for blood transfusions reduced by more than a 70%.
-
Tags
#Medicines